The extraction of nutrients and natural products has a long history - its origins can be traced all the way back to the ancient civilizations. At the time, the extracts were used for food, cosmetic and spiritual purposes, much like today. Archaeologists have found that more than 4,000 years ago, during the pharaonic dynasties, extractions were performed to obtain colors, aromas, and medicines.
During excavations in Iraq, they came across some pots dating back to 3500 BC, which were used to obtain the fragrances, active substances, and extracts they used during their religious ceremonies. The extraction pots were assembled in such a way that they could be used similarly to a "Soxhlet" device. In the Mediterranean region, preparing extracts is a long-standing tradition that was usually passed down from mother to daughter. This was how they were able to keep the secret of their fragrances and ancient medicines. One of the most revered early alchemists is without a doubt Mary of Alexandria, known as "Mary the Jewess", after whom the famous "bain-marie" (double boiler) is named. According to some rare writings, she managed to efficiently use solar energy to obtain extracts as early as 100 AD.
Today it is impossible to find a production process in the food, cosmetics, pharmaceutical, advanced materials and chemicals industries or the production of biofuels that does not include extraction processes. The extraction of natural active substances is considered "pure" by the general public when compared to the chemical industry. Researchers and the professional public tend to disagree with this, as the environmental impact can be much greater than it may seem at first glance. For example, essential oils are obtained from natural raw materials. However, traditionally this must be performed using only physical processes, which include alembic distillation with steam, water/steam or water, expression (cold pressing) or distillation with solvents. These processes are demanding due to high energy, solvent, water, and time consumption. Lipids, aromas, antioxidants, and dyes are still primarily obtained using Soxhlet extraction processes, which consist of repeated raw material rinsing with fresh solvent (usually hexane, acetone, dichloromethane and methanol). However, due to the complexity of the processes, used to obtain these solvents, it is still difficult to assess their overall impact on the environment and human health, even though the consumption of solvents and energy during the extraction process is very high. That is why organic chemists Paul T. Anastas and John C. Warner introduced the twelve principles of "green" chemistry in 1991. Not all green chemistry principles are suitable for extraction processes, as each extraction process is subject to specific chemical and physical laws. Taking into account the principles of green chemistry and green engineering, Chemat and colleagues therefore determined the six principles of green extraction:
Principle 1: Improvements in the selection of natural materials and use of easily renewable plant resources.
Principle 2: Use of alternative solvents, especially environmentally neutral supercritical fluids, water and agro-solvents (GRAS solvents).
Principle 3: Reduction of energy consumption with the possibility of energy recovery and use of innovative technologies.
Principle 4: Use of extraction process waste in the form of coproducts.
Principle 5: Reduction of the number of basic operations used to obtain the final products and favoring safe, robust and controlled processes.
Principle 6: The aim must be the production of non denatured and biodegradable extracts without impurities.
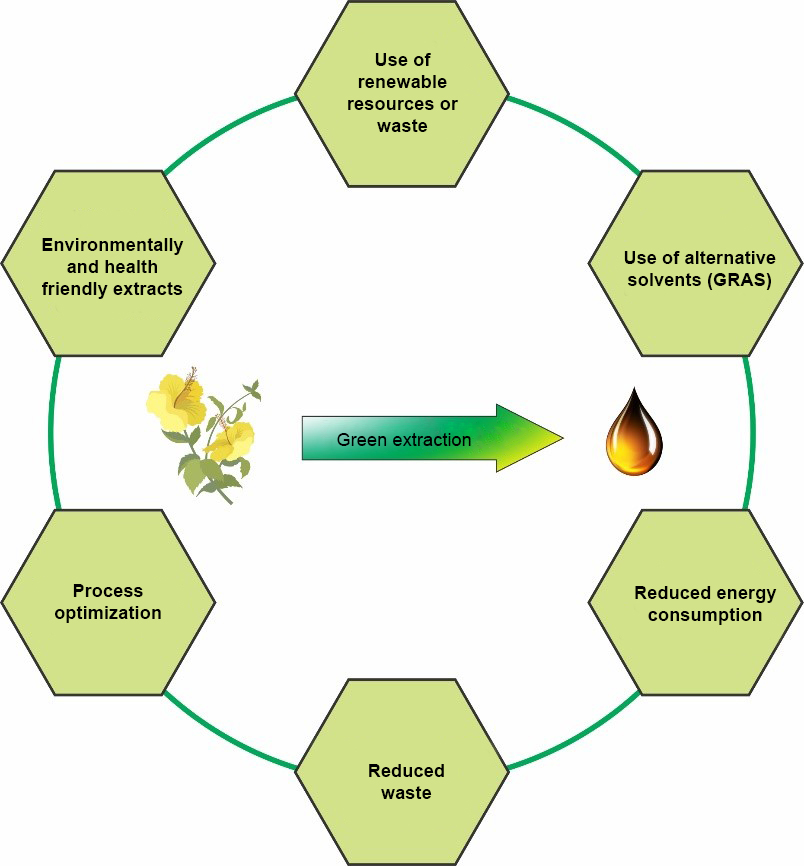
Figure 1: Six principles of green extraction
These principles are not rules but more guidelines, which are based on scientific discovery and can be safely applied at the industrial level.
Extraction processes for the production of bioactive substances with subcritical and supercritical carbon dioxide and water comply with all green extraction principles. If these bioactive substances can be extracted from agricultural residue and waste, the process also meets all industrial eco-symbiosis and circular economy requirements, as it enables the production of high value-added products from waste. Green extraction relies heavily on selecting the right solvent and process, which requires a lot of patience and attention to detail while planning.
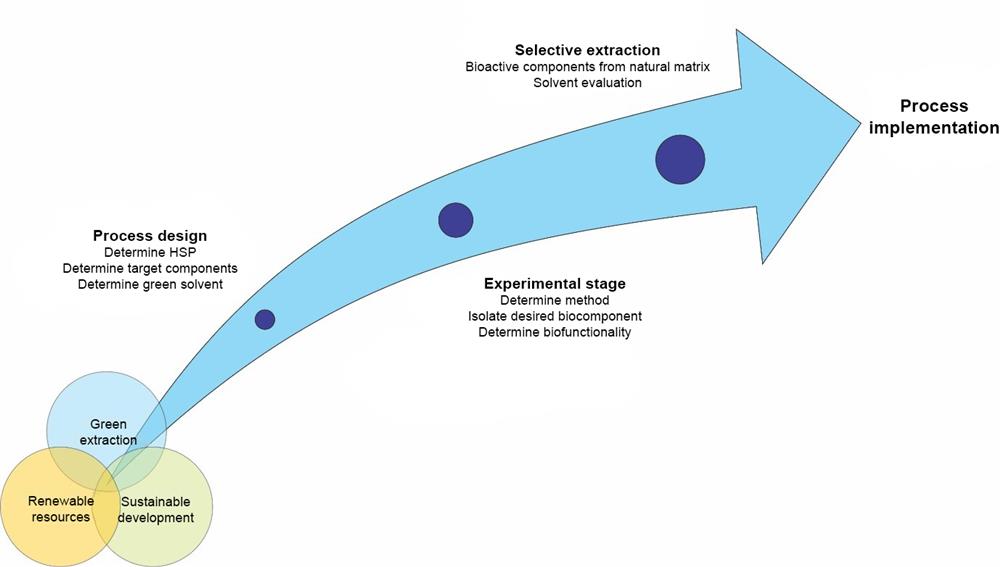
Figure 2: The development of a green extraction process
Modern technological processes that have already been developed and comply with these requirements include: subcritical and supercritical extraction processes, controlled pressure drop technology (DIC extraction), isobaric and nonisobaric extractions with liquid gases, extraction by extrusion, ultrasound-assisted extraction (UAE) and microwave-assisted extraction (MAE), pulsed electric field extraction (PEF) and high voltage electric discharge extraction (HVED), as well as all types of extractions at high and ultra-high pressures (PLE).
Plants are the most widespread natural entity on which we rely in search of pharmacological benefits. However, residues and waste are often neglected. The leaves and roots of potatoes and carrots, which are usually discarded, contain large amounts of phenolic compounds with antioxidant properties, such as chlorogenic acid and its derivatives, which is used in cosmetics as one of the main components of anti-cellulite and fat burning creams. Additionally, chlorogenic acid has antiviral and anti-inflammatory properties, it protects the heart and the blood vessels and accelerates injury healing. A similar example are grape seeds and those of other stone fruits. These residues are a rich source of anthocyanins, proanthocyanidins, phenolic acids and their derivatives, which are medically proven to protect the cardiovascular and nervous system, have an anti-inflammatory and antimicrobial effect and prevent the formation of tumors. Nature is an inexhaustible source of bioactive components, but it is important to bear in mind that while it creates the most effective medicines, it can also be used to obtain the deadliest toxins. Each process must be carefully planned in order to obtain an active substance that will have a beneficial effect on our metabolism.
Two green solvents that are often used for the extraction of bioactive substances from natural matrices are water and carbon dioxide.
What exactly are subcritical and supercritical fluids?
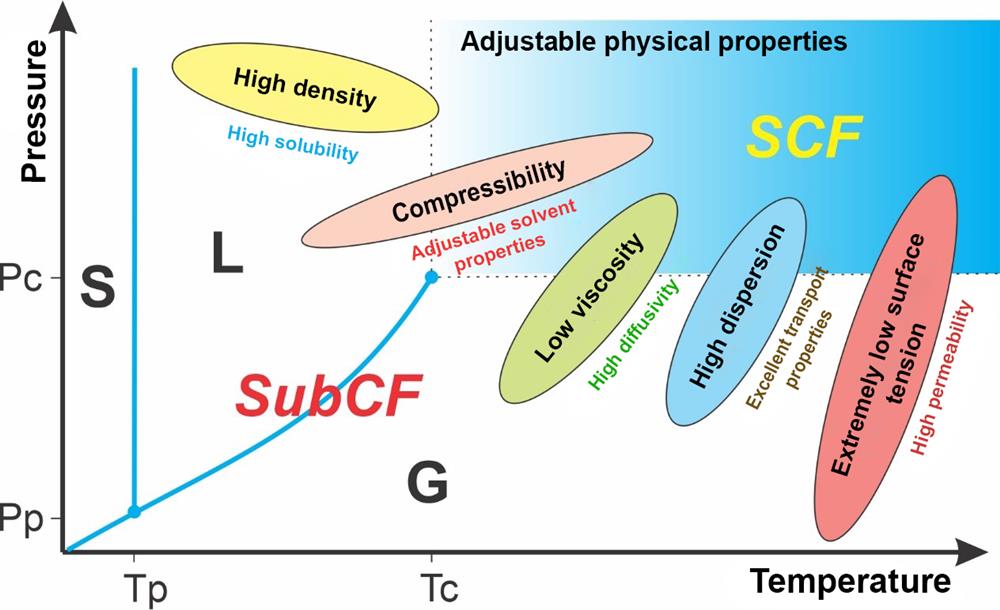
A subcritical fluid is a substance that is compressed at high pressures, but below its critical temperature point. Due to the high pressure it does not evaporate, but it does assume specific physical properties. The viscosity, density and, consequently, solubility of gases usually increase, while the dielectric constant of liquids changes.
| CO2 | H2O | EtOH | BZA | |
| Tc | 31.1 | 374 | 241 | 403 |
| Pc | 7.4 | 22.1 | 6.1 | 4.6 |
Figure 3: Properties of subcritical and supercritical fluids
A supercritical fluid is any substance at a temperature and pressure above its critical point. A substance in the supercritical state can diffuse through solids like a gas and dissolve substances like a liquid. Close to the critical point, even small fluctuations in pressure and temperature result in large changes in density, which enables precise regulation of many physical properties of the supercritical fluid. This is why they have become an important alternative for organic solvents in many laboratory and industrial processes, especially in extraction technologies.
The use of supercritical CO2 in extraction processes has increased quite rapidly; in fact, the extraction of food and natural products with supercritical or liquid CO2 is considered a relatively mature technology. A wide range of other uses of supercritical CO2 are also being studied in the context of modern technologies, including chemical reactions, polymer production and processing, semiconductor processing, powder production, environmental and soil remediation, and dry cleaning.
Supercritical fluids have thus found their place in numerous chemical processes, such as: supercritical extraction, particle size reduction in drug manufacturing, nanoparticle dosing, particle coating, production of micellar solutions, microemulsions, and amorphous drugs, processing of high-tech materials, supercritical fluid chromatography, application of oxidation processes, Friedel-Crafts alkylation, supercritical sterilization, drying of biological materials, synthesis of high-tech polymers, etc.
The applicability of supercritical fluids is directly related to their physicochemical properties. The ability to regulate the density with pressure changes ensures high solubility of the desired component and high extraction selectivity. Their low surface tension, low viscosity and ability to regulate diffusivity enable rapid fluid penetration into the pores and through cell walls, which in turn enables the extraction of internal cell material without tearing cell walls and, if necessary, their effective deactivation (for sterilization purposes). In certain cases, the same effects can also be achieved in the subcritical state near the critical point, where P > Pc and T < Tc.
Subcritical and supercritical extraction with carbon dioxide
If the process temperature increases, the transfer of substances improves and solvent consumption decreases (except in supercritical fluid-based extraction processes). This has a negative effect on process efficiency and often also on the stability of thermolabile bioactive compounds.
Extraction with subcritical and supercritical CO2 (SC-CO2) is a highly efficient green extraction process. Carbon dioxide is a nontoxic, nonflammable, noncorrosive, cheap and widely available gas that is easy to handle, which is why it is recognized in technological circles as a green solvent (GRAS). It enters supercritical conditions just above room temperature and at, in terms of technological conditions, relatively low pressures. It is chemically inert and, if properly purified, has a minimal effect on the quality of the extracted biocomponents, thus preserving their medicinal and functional properties. It dissolves fat-soluble (lipophilic) substances and can be completely removed from the final products by simply releasing the pressure.
Subcritical conditions can be used for the extraction of nonpolar temperature-sensitive components. The yields are usually small, but the quality of the products is good, as the extraction takes place in an inert atmosphere and at low temperatures. After releasing the pressure, the CO2 evaporates completely already at temperatures below 0 °C, which enables the extraction of natural extracts at low temperatures and without further processing. Good practices have been discovered in the extraction of volatile essential oils and carotenoids. In the food industry it is even more important to remove extracts and obtain a “purified” residue, such as the process of coffee decaffeination, which preserves the natural composition of other coffee components. Subcritical CO2 extraction is most often applied for the isolation of highly effective natural pharmacological components. Recently, there has been a rapid development of procedures for sterilizing pharmacological agents and medical devices, such as endoscopes, porous implants, intravenous fluids, liquid medications and liquid foods, where it is important to maintain an inert atmosphere and low process temperature. Subcritical extraction with CO2 is a fast-growing branch in the use of liquefied gases for the purposes of extraction and sterilization.
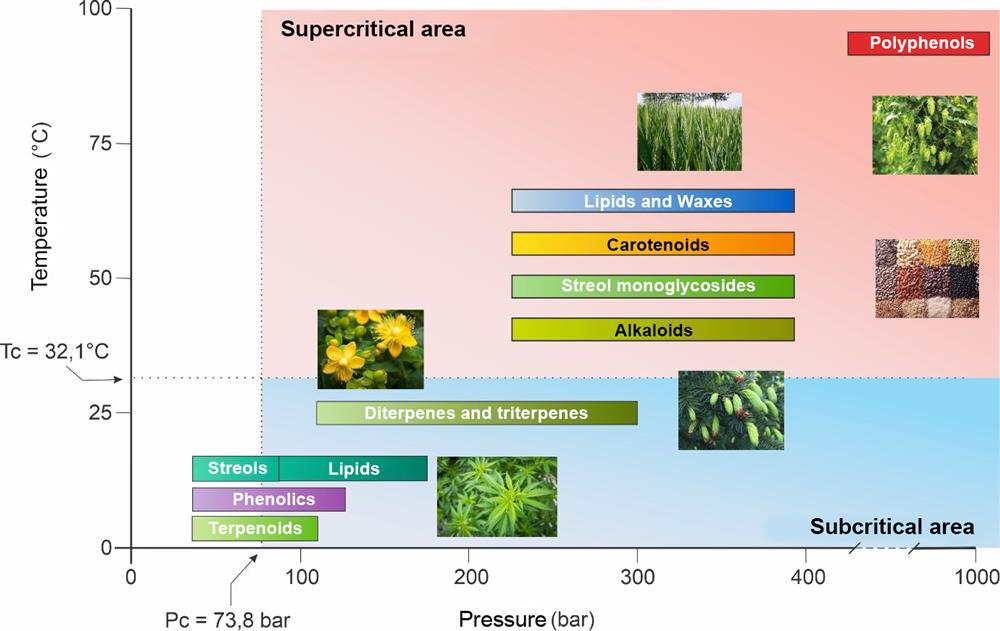
Figure 4: Possibilities of extraction with CO2 in subcritical and supercritical conditions
In contrast to subcritical extraction, supercritical extraction (SCE) is an already established separation process both at a laboratory (e.g. in the preparation of analytical samples) and an industrial scale (decaffeination, cannabinoid secretion, etc.). Due to their high efficiency and cost-effectiveness, SCE processes are widely used in the food and pharmaceutical industries, as well as in other sectors that include the fields of toxicology, chemistry, environment, textiles, petrochemistry and polymers. Great advances in the supercritical technology over the last three decades have stimulated the extraction of natural extracts from natural materials, including plants and their residues, algae and microalgae. The advantages of SCE are high selectivity, short extraction times and the possibility of using nontoxic organic solvents that can be completely removed from the final product. The efficiency of the processes is based on the dynamic control of the physical properties of solvents, such as density, diffusivity, dielectric constant and viscosity. All these properties can be easily regulated in the supercritical range by changing the pressure and temperature. Under these supercritical conditions, a substance exists in a state between a liquid and a gas; its density is similar to a liquid, while the viscosity, surface tension, and thermal properties are more gas-like.
| Property |
Density [kg/m3] |
Viscosity [cP] |
Diffusivity [mm2/s] |
| Gas |
1 | 0.01 | 1-10 |
| Supercritical fluid | 100-800 | 0.05-0.1 | 0.01-0.1 |
| Liquid | 1000 | 0.5-1.0 | 0.001 |
Table 1: Comparison of typical density, viscosity and diffusivity values
The supercritical state of a fluid is a state in which a liquid and a gas are equivalent to each other, while the physical state does not change despite a change in pressure or temperature.
The number of applications in the food and pharmaceutical industries has increased exponentially in the last few years, as an incredible amount of effort has been put into research and the commercialization of obtaining "natural" extracts for use in functional foods and medicines. Various studies show that oils made from pits and nuts are often obtained by supercritical extraction, mainly because they are rich in bioactive components, few in quantity, and therefore very valuable. This process enables the extraction of carotenoids, β-carotene and lycopene, as well as phytosterols, phospholipids, squalene and Q-enzyme 10, which lower cholesterol levels and have antioxidant properties. Despite the low solubility of the phenolic substances in SC-CO2, extractions of these substances under supercritical conditions are extensively carried out mainly due to their pharmacological value. Newer extraction methods are being developed using ultra-high pressures over 1000 bar, where CO2 starts becoming polar, using multistage extractions, cosolvents and compression-relaxation cycles. Supercritical technology, especially in conjunction with CO2, is used in modern process technology for sterilization without affecting the organoleptic properties of bioactive substances and the quality of the components that would otherwise be destroyed if conventional thermal or chemical processes were used.
The driving force of the extraction processes is based on the interaction between the solvent and the dissolved component, as well as the mass transfer. The SCE has proven to be a great technique for extracting bioactive components from natural materials and their residues mainly due to relatively short extraction times, low extraction temperatures, and the purity of the obtained extract, although good knowledge of the solubility product is essential in order to perform an efficient extraction.
Supercritical CO2 has excellent solvation properties that can be effectively controlled by regulating the temperature and pressure, which greatly affects the density of CO2 and, consequently, the strength of the solvent. Higher pressure values increase the density of the SC-CO2, which becomes similar to the density of liquids. Consequently, the solute and the solvent are more likely to interact, which leads to a large increase in solubility. Raising the temperature, on the other hand, reduces the SC-CO2 density and at the same time increases the vapor pressure of the solute. The net effect of these two conflicting factors dictates a change in solubility. At relatively low pressures near the critical point, the density of SC-CO2 and the solubility decrease as the temperature increases. However, at higher pressures the effect of vapor pressures is shown, where temperature-based density changes are relatively small and as the temperature increases, so does the solubility. What occurs as a consequence of these transitions is the cross-behavior of the solubility isotherms. Solubility is also affected by the physicochemical properties of the solute, such as its molecular weight, vapor pressure, and ability to interact with the solvent (e.g. hydrogen bonding, etc.).
As is shown in Figure 4, the solubility of the component to be extracted decreases as its molecular weight and vapor pressure increase. As a rule, SC-CO2 at lower densities can be used to dissolve nonpolar substances with low molecular weight and higher vapor pressure, while more polar and less volatile substances require denser solvents. The selectivity of the extraction process is easily achieved by regulating the pressure and temperature, which is the main advantage of supercritical extractions, as they enable us to obtain pure products without unwanted impurities, as well as to completely remove the solvent with a simple pressure relief. This significantly reduces the cost of subsequent final product purification.
Although CO2 is primarily used for the extraction of nonpolar compounds, the polarity of the supercritical CO2 can be easily regulated by the addition of an easily miscible polar modifier (e.g. ethanol). The solvent accelerates the extraction by establishing hydrogen bonds with the substance to be extracted. It forms dipole couplings and charge-transfer complexes and increases solvent density. Edible ethanol is generally used as a cosolvent in pharmacology and the food industry. Other cosolvents are used when the quality and price of the product can justify the cost of their removal.
In order to successfully and efficiently carry out the biocomponent extraction, other parameters must also be taken into account, including pretreatment of the extracted substance, particle size, temperature, pressure, flow, solvent delivery method, and the extracted product-solvent ratio. These parameters can have a significant influence on the yield and subsequent treatment of the extract.
Škrlj extraction system MoSES
The Škrlj SCE MoSES extraction system is designed for the extraction of compounds from solid or semisolid substances. By precisely regulating the pressure and temperature, CO2 can be used to selectively extract a specific component. The system can operate in the subcritical and supercritical region of a chosen solvent, with pressure and temperature values up to 400 bar and 100 °C. In this system, configured to purify and collect the extracted compounds, the feed material is loaded into an extraction vessel. Depending on the application, the feed material is soaked (static process) or continuously rinsed (dynamic process). The solvent used is CO2 in its subcritical or supercritical state with the addition of a cosolvent, if the nature of the extraction requires it. Sub- and supercritical conditions in the extraction vessel are established using a cooled membrane pump and a heater, which is connected in series. The pressure in the extraction vessel is maintained using a pressure regulator. The automatic back pressure regulator (ABPR), located between the extraction vessel and the cyclone separators, enables a controlled reduction of the CO2 pressure. Using the CO2 flow, the dissolved component is occasionally or continuously transferred from the extraction vessel to one or more cyclone separators, depending on the application. After the ABPR, the system pressure decreases, which is why the CO2 loses its solvent power. When the manual back pressure regulators (MBPR) are set correctly, the extracted material falls out of the solution and into the cyclone separators according to the solubility product of the individual components in the extract, which allows us to selectively separate the extract into individual components. The purified CO2 is redirected to the cooled CO2 recycler. The diagram below shows the key components that make up the system flow of the MoSES device, configured with a cosolvent pump, two extraction vessels, and three cyclone separators.
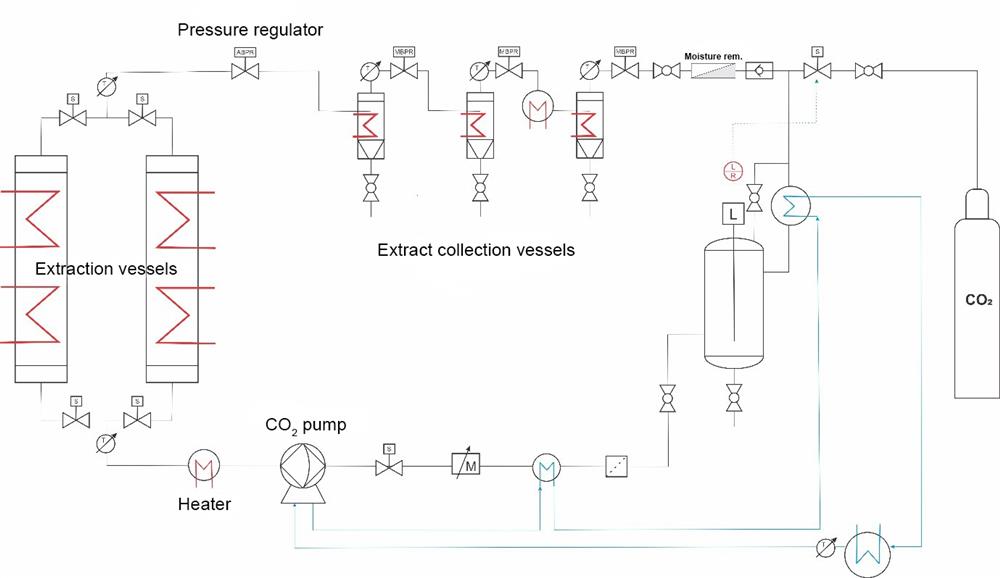
Figure 5: Operation diagram of the Škrlj SCE MoSES extractor
